Background
Patients with multiple myeloma (MM) have worsened HRQoL that deteriorates with progression or with increasing lines of therapy (Fonseca et al. Clin Lymphoma Myeloma Leuk. 2023). Assessing HRQoL in patients with RRMM provides insights on the full benefit of treatment. LINKER-MM1 (NCT03761108) is an ongoing open-label, multicenter Phase 1/2 dose-escalation and dose-expansion trial evaluating linvoseltamab, a B-cell maturation antigen (BCMA)×CD3 bispecific antibody that targets CD3 on T-cells and BCMA on myeloma cells resulting in T-cell-mediated cytotoxicity. Results have demonstrated promising efficacy and generally manageable safety for linvoseltamab in patients with RRMM (Lee et al. ASCO. 2023). Patient-reported outcomes (PRO) from the Phase 1 portion have shown improvements in quality of life (QoL) and pain symptoms. We report PRO for the combined Phase 1/2 cohort receiving the linvoseltamab 5-25-200 mg dosing regimen.
Methods
Patients in LINKER-MM1 (June 7, 2023 data cut-off with a July 20, 2023 data extraction) who were enrolled to receive the 5-25-200 mg dosing regimen during Phase 1 (n=12) and Phase 2 (n=105) were included in the analysis. Patients were ≥18 years of age with RRMM after triple-class exposure to a proteasome inhibitor, immunomodulatory drug, and an anti-CD38 antibody. Linvoseltamab was administered at Week 1 (5 mg), Week 2 (25 mg), and Week 3 (200 mg), followed by weekly dosing through Week 14 (Phase 2) or Week 16 (Phase 1), then every 2 weeks thereafter until progression. Patients in Phase 2 who achieved a very good partial response by Week 24 could transition to every 4 week dosing from Week 24. The PRO instruments included the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Core 30 (QLQ-C30), EORTC Quality of Life Questionnaire Multiple Myeloma module (QLQ-MY20), and European Quality of Life 5 Dimensions 3 Level questionnaire (EQ-5D-3L) administered at baseline, Week 4, and every 4 weeks thereafter. Six scales across the three instruments were pre-specified in the clinical statistical analysis plan. We present analyses of the first 36 weeks of treatment. Least squares (LS) mean change from baseline to each assessment visit in PRO scores was estimated using a mixed effects model for repeated measures. Clinically meaningful improvements for EORTC QLQ-C30 were defined based on the minimally important difference (MID) of ≥10 points, with higher scores indicating better global health status (GHS)/QoL and physical functioning (PF). For EORTC QLQ-C30 fatigue (MID ≥10 points) and EORTC QLQ-MY20 Disease Symptoms (MID ≥16 points) and Side Effects of Treatment symptom scales (MID ≥6 points), lower scores indicate a lower symptom burden. For EQ-5D-3L visual analog scale (VAS), changes ≥12 points were considered clinically meaningful, with higher scores indicating better health status. LS mean change from baseline was considered statistically significant if the 95% confidence interval (CI) did not cross 0. No adjustment for multiplicity was performed, hence all statistical significance is nominal.
Results
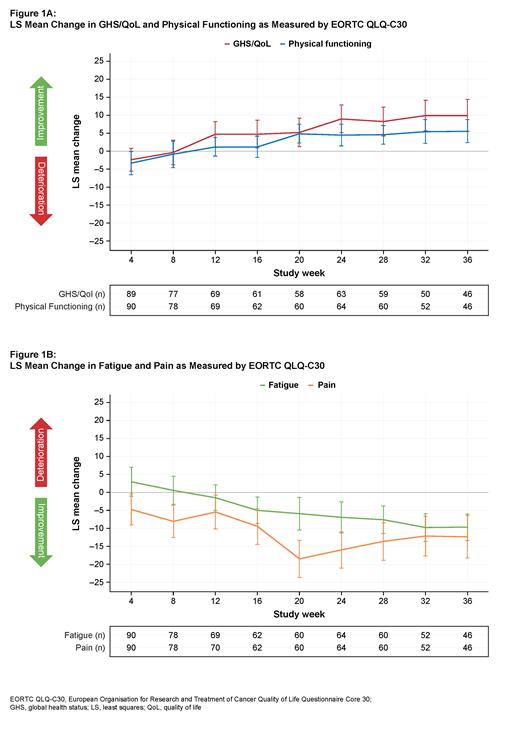
PRO completion rates were high with ≥85% for the majority of time points among expected visits. Estimated mean changes from baseline by time point were statistically significant for GHS/QoL from Week 12 up to Week 36, with a LS mean change of 9.97 (95% CI 5.51, 14.43) at Week 36 (Figure 1A). Statistically significant improvements were observed for fatigue at Week 16 up to Week 36 with a LS mean change of −9.66 (95% CI −13.35, −5.98) at Week 36. Notably, both statistically significant and clinically meaningful improvements were observed for pain at Week 20 (−18.43; 95% CI −23.54, −13.32) through Week 36 (−12.28; 95% CI −18.15, −6.42) (Figure 1B). Statistically significant improvements were observed in PF at Week 20 up to Week 36 (Figure 1A) and most assessment time points for the EORTC QLQ-MY20 Disease Symptoms and Side Effects of Treatment scales during the 36-week period, although these changes did not reach the MID thresholds for clinically meaningful improvement. Scores on the EQ-5D-3L VAS were maintained from baseline throughout the 36-week period.
Conclusions
During 36 weeks of treatment, improvements in measures of HRQoL, including pain, were reported among patients with RRMM receiving linvoseltamab. These PRO findings support a favorable benefit-risk profile of linvoseltamab consistent with clinical results.
Disclosures
Richter:Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy; Sanofi: Membership on an entity's Board of Directors or advisory committees; Bristol-Meyers-Squibb: Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Dhodapkar:Sanofi: Membership on an entity's Board of Directors or advisory committees; Lava Therapeutics: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees. Lee:Bristol Myers Squibb: Consultancy, Research Funding; Genentech: Consultancy; GlaxoSmithKline: Consultancy, Research Funding; Sanofi: Consultancy; Pfizer: Consultancy; Monte Rosa Therapeutics: Consultancy; Takeda Pharmaceuticals: Consultancy, Research Funding; Allogene Thereapeutics: Consultancy; Regeneron: Consultancy, Research Funding; Amgen: Research Funding; Janssen: Consultancy, Research Funding; Celgene: Consultancy. Houde:Janssen: Research Funding; Alexion, AstraZeneca Rare Diseases: Research Funding; BMS: Consultancy, Research Funding; Alnylam, Amgen, CaelumBiosciences, Celgene, Intellia,Janssen, Prothena, Regeneron: Consultancy; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees. Shah:Janssen: Consultancy; Bristol Myers Squibb: Consultancy. Baz:Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Regeneron: Research Funding; GSK: Honoraria; AbbVie: Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; HIKMA Cancer Network: Honoraria; Curio Science: Honoraria; AHOMPR: Honoraria; ASH: Honoraria. Namburi:Janssen: Honoraria; Genentech: Honoraria; BMS: Honoraria. Pianko:Pfizer: Consultancy, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Regeneron: Research Funding; BMS: Research Funding; Sanofi: Honoraria, Research Funding; Nektar: Research Funding; Abbvie: Research Funding. Ye:Celgene: Honoraria; Novartis: Research Funding; Pfizer: Research Funding; MingSight: Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria; Regeneron: Honoraria; GlaxoSmithKline: Research Funding; Genmab: Research Funding; Janssen Scientific Affairs: Honoraria; Janssen: Consultancy. Lentzsch:Pfizer: Consultancy; Karyopharm Therapeutics: Membership on an entity's Board of Directors or advisory committees; Oncopeptide: Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Consultancy, Membership on an entity's Board of Directors or advisory committees; Alexion Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Bristol Meyers Squibb: Membership on an entity's Board of Directors or advisory committees; Caelum Biosciences: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: January 1, 2041; Sanofi: Research Funding; Regeneron: Honoraria; Clinical Care Options: Honoraria; Takeda: Membership on an entity's Board of Directors or advisory committees. Munder:GlaxoSmithKline: Consultancy; Takeda: Consultancy, Honoraria; Sanofi: Consultancy; Incyte: Research Funding; BMS: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Lopez:GSK: Consultancy, Speakers Bureau; Menarini: Consultancy, Speakers Bureau; Sanofi: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Incity: Consultancy, Speakers Bureau; Roche: Consultancy, Speakers Bureau; Gilead: Consultancy, Speakers Bureau; BMS: Consultancy, Research Funding, Speakers Bureau; Janssen: Consultancy, Research Funding, Speakers Bureau. DeVeaux:Regeneron: Current Employment, Current holder of stock options in a privately-held company. Ivanescu:IQVIA: Current Employment. Rodriguez Lorenc:Regeneron: Current Employment, Current holder of stock options in a privately-held company. Kroog:Regeneron Pharmaceuticals, Inc.: Current Employment, Current holder of stock options in a privately-held company. Houvras:Regeneron: Current Employment. Inocencio:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in publicly-traded company. Chi:Regeneron: Current Employment, Current holder of stock options in a privately-held company. Harnett:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in publicly-traded company. Ma:Regeneron: Current Employment, Current equity holder in publicly-traded company. Jagannath:Bristol Myers Squibb: Consultancy; Janssen: Consultancy; Sanofi: Consultancy; Caribou Biosciences: Consultancy; Takeda: Consultancy; Regeneron: Consultancy; DMC: Membership on an entity's Board of Directors or advisory committees; Mount Sinai Hospital: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal